Autophagy and Neuronal Health: Powering Brain Health: How Autophagy Protects Neurons By
Powering brain health: How autophagy protects neurons by – Autophagy, meaning “self-eating,” is a fundamental cellular process crucial for maintaining neuronal health. It involves the degradation and recycling of damaged cellular components, ensuring cellular homeostasis and survival. This process is vital for neurons, which are long-lived cells with high metabolic demands and limited regenerative capacity. Impaired autophagy is implicated in various neurodegenerative diseases, underscoring its importance in brain health.
Autophagic Processes in Neuron Protection, Powering brain health: How autophagy protects neurons by
Autophagy operates through several molecular pathways to eliminate damaged cellular components within neurons. These pathways involve the formation of autophagosomes, double-membraned vesicles that engulf targeted materials. These autophagosomes then fuse with lysosomes, organelles containing degradative enzymes, resulting in the breakdown of the enclosed components. This process is essential for removing misfolded proteins, damaged organelles like mitochondria, and aggregated proteins that can be toxic to neurons.
Types of Autophagy and Their Roles
Three main types of autophagy contribute to neuronal protection: macroautophagy, microautophagy, and chaperone-mediated autophagy. Macroautophagy is the most prevalent form, involving the formation of large autophagosomes. Microautophagy involves the direct engulfment of cytoplasmic components by lysosomes. Chaperone-mediated autophagy selectively targets specific proteins for lysosomal degradation. Each type plays a distinct role in maintaining neuronal health, and their dysregulation contributes to neurodegeneration.
Factors Influencing Autophagic Activity
Several factors modulate autophagic activity in neurons, influencing their health and susceptibility to neurodegenerative diseases. Some factors promote autophagy, while others inhibit it. This delicate balance is critical for maintaining neuronal homeostasis.
| Factor | Type | Mechanism of Action | Impact on Neuronal Health |
|---|---|---|---|
| Exercise | Promoting | Increases cellular energy demand, stimulating autophagic flux. | Improved mitochondrial function, reduced protein aggregation. |
| Caloric Restriction | Promoting | Activates cellular stress response pathways, enhancing autophagy. | Increased clearance of damaged organelles, reduced oxidative stress. |
| Resveratrol | Promoting | Activates sirtuins, which regulate autophagy genes. | Improved mitochondrial biogenesis, reduced inflammation. |
| Aging | Inhibiting | Reduced autophagic capacity due to decreased lysosomal function and impaired autophagosome formation. | Accumulation of damaged proteins and organelles, increased oxidative stress. |
| Inflammation | Inhibiting | Inflammatory mediators interfere with autophagic pathways. | Increased neuronal damage and apoptosis. |
| Oxidative Stress | Inhibiting | Reactive oxygen species damage autophagic machinery and impair lysosomal function. | Increased protein aggregation and neuronal dysfunction. |
Autophagy Dysfunction in Neurodegenerative Diseases
Impaired autophagy plays a significant role in the pathogenesis of several neurodegenerative diseases. The accumulation of misfolded proteins and damaged organelles, due to deficient autophagy, contributes to neuronal dysfunction and cell death.
Autophagy and Alzheimer’s Disease
In Alzheimer’s disease, impaired autophagy contributes to the accumulation of amyloid-beta plaques and tau tangles, hallmarks of the disease. This accumulation leads to synaptic dysfunction and neuronal loss.
Flowchart: Autophagy Impairment in Alzheimer’s Disease
A flowchart illustrating the progression from autophagy impairment to Alzheimer’s disease would show the following steps: 1. Genetic or environmental factors leading to reduced autophagy. 2. Accumulation of amyloid-beta and tau proteins. 3. Formation of senile plaques and neurofibrillary tangles. 4. Synaptic dysfunction and neuronal loss. 5. Cognitive decline and Alzheimer’s disease symptoms.
Therapeutic Strategies Targeting Autophagy
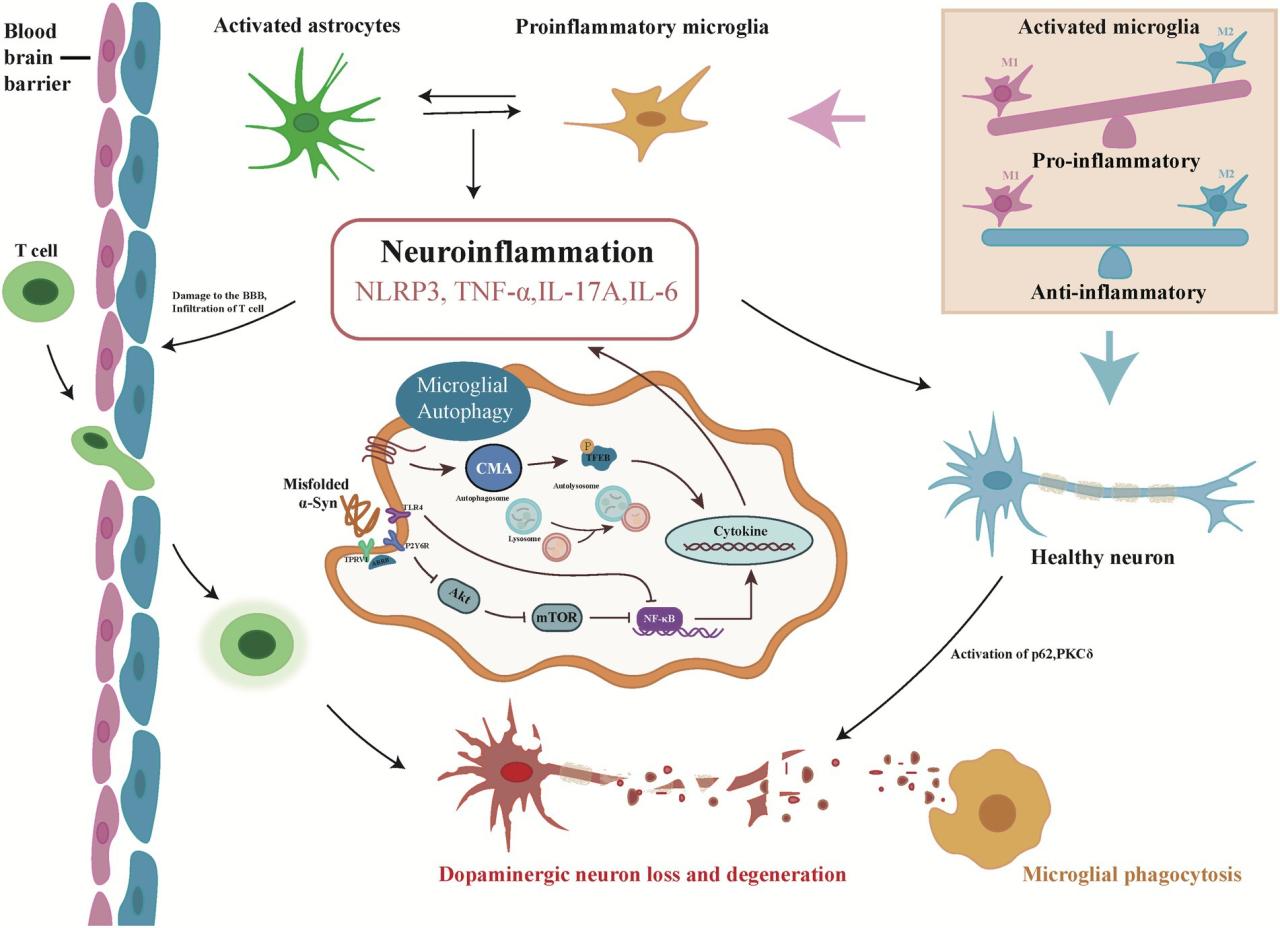
Enhancing autophagy presents a promising therapeutic strategy for neurodegenerative diseases. Several approaches are being investigated to modulate autophagic activity and protect neurons.
- Pharmacological agents: Some compounds, like rapamycin (an mTOR inhibitor), can stimulate autophagy. Mechanism of action: Rapamycin inhibits mTOR, a central regulator of autophagy, leading to increased autophagic flux. Potential benefits: Improved clearance of misfolded proteins and damaged organelles. Limitations: Potential side effects, including immunosuppression.
- Lifestyle modifications: Exercise and caloric restriction can enhance autophagy. Mechanism of action: These interventions activate cellular stress response pathways, promoting autophagy. Potential benefits: Improved mitochondrial function, reduced oxidative stress. Limitations: Requires adherence to a specific lifestyle.
- Gene therapy: Strategies aimed at correcting genetic defects affecting autophagy pathways are under development. Mechanism of action: Restoration of normal autophagy function. Potential benefits: Long-term correction of autophagy defects. Limitations: Challenges in gene delivery and potential off-target effects.
Future Directions in Autophagy Research
Despite significant advances, many questions remain unanswered regarding the role of autophagy in neuronal protection and neurodegenerative diseases. Further research is needed to fully elucidate the complex interplay between autophagy and neuronal health.
Visual Representation of Autophagy Modulation
An image depicting autophagy modulation as a therapeutic strategy could show a neuron with damaged components (e.g., misfolded proteins, damaged mitochondria) surrounded by autophagosomes. Arrows would indicate the process of engulfment and degradation of these components. The healthy neuron with reduced damaged components and restored function would be contrasted with a neuron where autophagy is impaired, showcasing the accumulation of damaged components and subsequent neuronal dysfunction.
